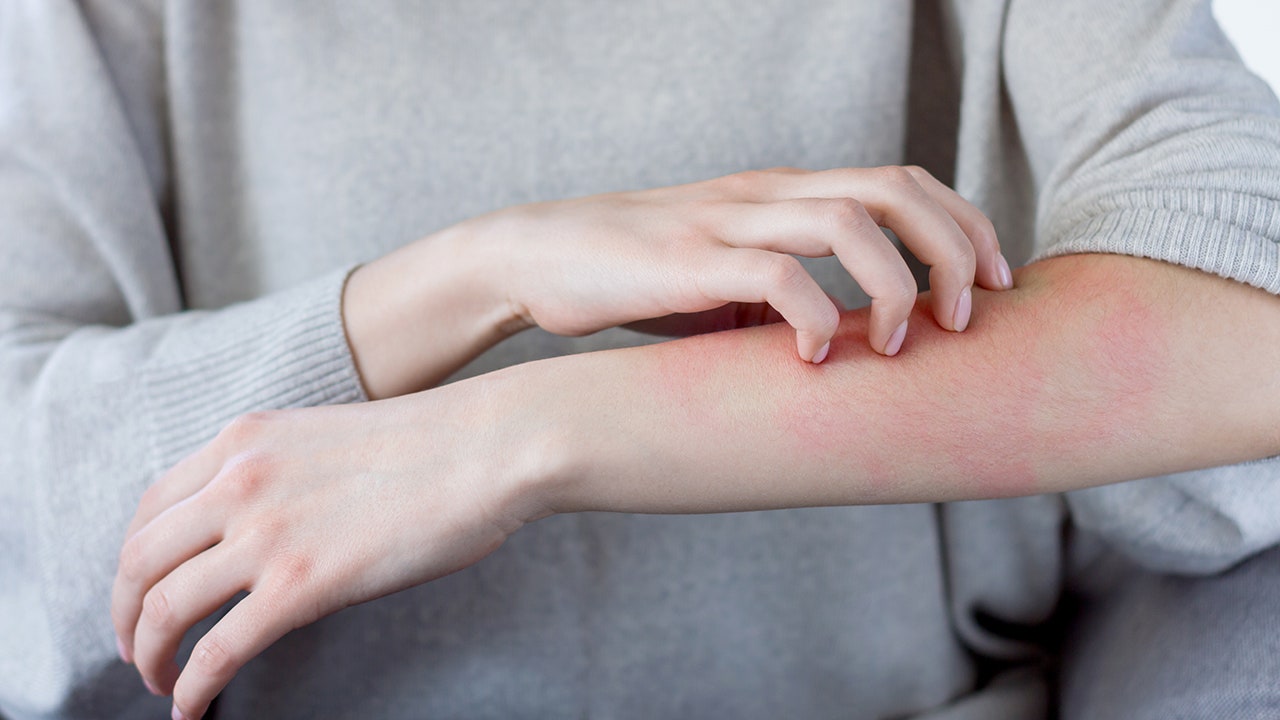
Some receipts for Moderna’s COVID-19 vaccine developed a delayed skin reaction to the vaccine days after receiving it, several doctors wrote in a letter published in the New England Journal of Medicine this week.
In the letter, doctors detailed 12 cases of late skin reactions that appeared about four to 11 days after the first dose of Moderna vaccine, with an average of eight days. About half of the patients also developed a skin reaction after the second dose, although it was less severe.

The doctors observed that all recipients of the vaccine started to receive the second dose.
(iStock)
Most were treated with antihistamines and ice, but some patients required steroid treatments that were prescribed in the form of threads or pills. Most skin rashes disappear after four to five days.
The rashes were harmless, but could be mistaken for an infection, which resulted in the unnecessary use of antibiotics in at least one patient who developed this reaction, they wrote in the letter.
CDC DELAY GUIDANCE FOR VACCINATED POPULATION OF COVID-19
“Doctors may not be prepared to deal with late local reactions to the mRNA-1273 vaccine. Given the expansion of mass vaccination campaigns around the world, these reactions can raise concerns among patients and requests for evaluation. These reactions have not been consistently recognized, guidance on the second dose of the vaccine has varied, and many patients have received antibiotics unnecessarily, “they wrote.” We hope that this letter will encourage additional reports and communications on the epidemiological characteristics, causes and implications of these skin reactions. delayed, as this information can calm patients’ concerns, encourage completion of vaccination and minimize unnecessary use of antibiotics. “
Late skin reactions were observed in Moderna’s large clinical trial with its vaccine, occurring in less than 1% of prescriptions after the first dose and only in 0.2% after receiving the second dose.
Doctors noted that all recipients of the vaccine began to receive the second dose.
“Given that neither local injection site reactions nor delayed-type hypersensitivity reactions are contraindications for subsequent vaccination, all 12 patients were encouraged to receive the second dose and completed their vaccination schedule with mRNA-1273” , they wrote.
CLICK HERE FOR FULL CORONAVIRUS COVERAGE
Speaking to Bloomberg, Kimberly Blumenthal, lead author of the article and co-director of the clinical epidemiology program at Massachusetts General Hospital in Boston, asked those who may have a delayed skin reaction after the first dose to ensure they receive the second, like this one. specific reaction is not a danger, she said.
“Our goal was to show how dramatic they can be, while at the same time nobody has had them more severe with dose 2,” she said. “Many of the patients I took care of with this were concerned about them. Is this an infection? (No!) Does that mean I can’t take dose 2? (No!) Will this happen with dose 2? (Not necessarily!) ,” she said.
“This is a nuisance, but it is not dangerous,” added Blumenthal.