Visit the News Center
Measuring mitochondrial DNA can predict who will need ICU care, intubation
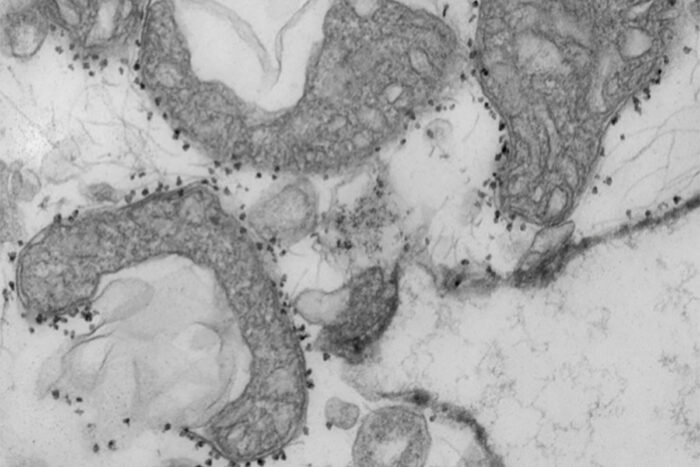
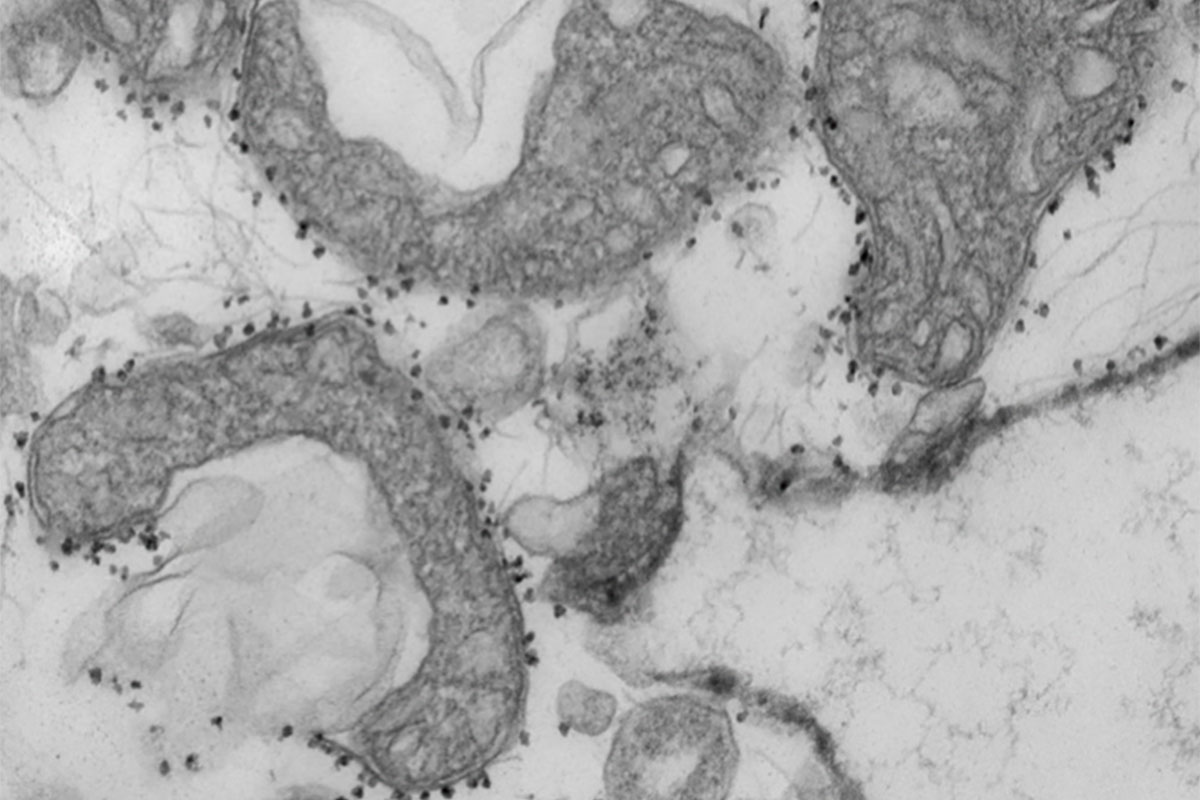
A new study from the University of Washington School of Medicine in St. Louis suggests that measuring mitochondrial DNA in the blood of patients with COVID-19 may help predict which patients are most at risk for serious illness, requiring more intensive care. Mitochondrial DNA levels are a measure of tissue damage. In the photo are damaged mitochondria (dark gray areas) released from human lungs. The small dark spots around the mitochondria are magnetic spheres that carry antibodies used to isolate and study harmful mitochondria that have been released from dying tissues.
One of the most irritating aspects of the COVID-19 pandemic is the doctors’ inability to predict which newly hospitalized patients will develop serious illness, including complications that require the insertion of a breathing tube, kidney dialysis or other intensive care. Knowing the patient’s age and underlying medical conditions can help predict these results, but there are still surprises when younger and apparently healthier patients experience serious complications that can lead to death.
Now, scientists at the University of Washington School of Medicine in St. Louis have shown that a relatively simple and quick blood test can predict – within a day of hospital admission – which patients with COVID-19 are at the highest risk for complications. serious or death.
The study, published Jan. 14 in JCI Insight, involved nearly 100 patients recently admitted to the hospital with COVID-19.
The blood test measures levels of mitochondrial DNA, a unique type of DNA molecule that normally resides within the cell’s energy factories. The mitochondrial DNA that leaves the cells and goes into the bloodstream is a sign that a specific type of violent cell death is occurring in the body.
“Doctors need better tools to assess the status of patients with COVID-19 as early as possible because many of the treatments – such as monoclonal antibodies – are scarce and we know that some patients will improve without intensive treatment,” said co-author Andrew E. Gelman, PhD, Jacqueline G. and William E. Maritz President with Immunology and Oncology in the Department of Surgery.
“There is so much that we still don’t understand about this disease,” he added. “In particular, we need to understand why some patients, regardless of their age or underlying health in some cases, enter this spiral of hyperinflammatory death. Our study suggests that tissue damage may be one of the causes of this spiral, since the mitochondrial DNA that is released is itself an inflammatory molecule ”.
The researchers said the test could serve as a way to predict the severity of the disease, as well as a tool to better design clinical trials, identifying patients who may, for example, benefit from specific investigational treatments. They also said they would like to assess whether the test could serve as a way to monitor the effectiveness of new therapies. Presumably, effective treatments would reduce the levels of mitochondrial DNA.
“We will need larger studies to verify what we found in this study, but if we could determine in the first 24 hours of admission whether a patient is likely to need dialysis or intubation or medication to prevent his blood pressure from falling too much, which would change the way we do the patient screening, and can change the way we treat them much earlier in the course of the disease, ”said co-author Hrishikesh S. Kulkarni, MD, assistant professor of medicine.
The researchers, including co-authors Davide Scozzi, MD, PhD, a scientist on the team, and Marlene Cano, PhD, a postdoctoral researcher, evaluated 97 patients with COVID-19 at Barnes-Jewish Hospital, measuring their levels of Mitochondrial DNA on the first day of hospitalization. They found that mitochondrial DNA levels were much higher in patients who were eventually admitted to the ICU, intubated or died. The researchers found that this association occurred regardless of the patient’s age, sex and underlying health conditions.
On average, mitochondrial DNA levels were about ten times higher in patients with COVID-19 who developed severe lung dysfunction or eventually died. Those with high levels were almost six times more likely to be intubated, three times more likely to be admitted to the ICU and almost twice more likely to die compared to those with lower levels.
In addition, the test predicted results as well or better than the existing inflammation markers currently measured in patients hospitalized with COVID-19. Most other markers of inflammation measured in patients with COVID-19, including those still under investigation, are general markers of systemic inflammation, rather than specific inflammation for cell death, according to the researchers.
“Viruses can cause a type of tissue damage called necrosis, which is a violent and inflammatory response to infection,” said Gelman. “The cell breaks down, releasing the content, including mitochondrial DNA, which in itself leads to inflammation. In patients with COVID-19, there was anecdotal evidence of this type of cellular and tissue damage to the lung, heart and kidney. We think it is possible that measurements of mitochondrial DNA in the blood are an early sign of this type of cell death in vital organs. “
The researchers also emphasized that the test is quick and simple to perform in most hospital settings because it uses the same machine that processes the standard PCR test for COVID-19. The method they developed allows the levels of mitochondrial DNA to be quantified directly in the blood. Without requiring intermediate steps to extract DNA from the blood, the technique returned results in less than an hour.
Before applying for approval from the Food and Drug Administration (FDA), scientists need to verify that the test is accurate in a larger multicenter study. They have plans to expand the search to more locations.
The study used samples obtained from the COVID-19 biorepository of the School of Medicine, developed by co-authors Jane O’Halloran, MD, PhD, assistant professor of medicine; Charles Goss, PhD, biostatistics instructor; and Phillip Mudd, MD, PhD, assistant professor of emergency medicine.