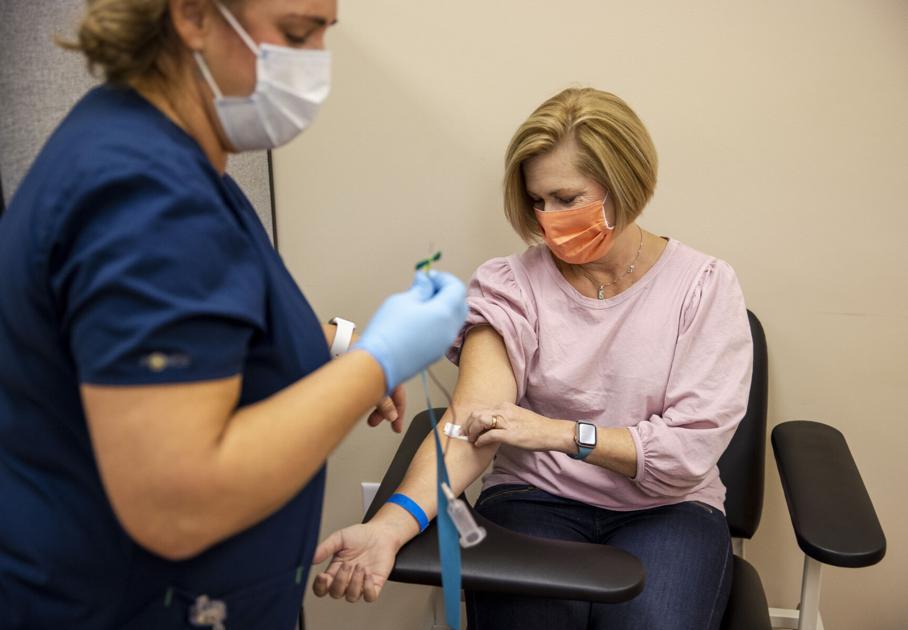
Inside a small brick building in North Charleston, where a global search was underway, Ginger Bridges pulled the neckline down a few inches to expose his left shoulder.
It took more than an hour for the 51-year-old pharmacist to get to a chair inside the Clinical Trials of South Carolina lab. The consent paperwork, brief physical exam, blood collection, vital signs, medical issues and a COVID-19 tear induction test came first.
The laboratory, which participates in phase three of the clinical trials for AstraZeneca’s COVID-19 vaccine, was full of boxes, flasks and refrigerators. Outside, two indefinable trailers parked in their parking lot as part of Operation Warp Speed, an effort to research and produce a mass of vaccines to fight a virus that killed nearly 300,000 people in the United States alone.
A nurse approached with a syringe.
Bridges inhaled. She wondered out loud about her chances that the clear liquid inside contained the vaccine, not a placebo.
“It’s two in three, right?” she asked the nurse, Cathie Zimmerman, who nodded.
An algorithm randomizes which two-thirds of the participants receive the vaccine.
“Please be authentic,” said Bridges.
The fine needle plunged into his shoulder.
On Thursday, Bridges became known in the laboratory as participant # 677, which means that the test site was almost halfway through its goal of enrolling 1,500 people. The SC Clinical Trials have seen around 50 participants a day and have actively recruited more, especially people of color.
Research manager Nathelia O’Banner handed Bridges a beaded bracelet that said, “Win Covid.” Bridges would return in exactly 29 days for his second dose.
Outside, under the bright sun, she rubbed her shoulder, but not because it hurt. She wasn’t sorry about anything, not yet.

Small price to pay
As Bridges returned home to Johns Island, she knew it was probably too early for body clues that could tell if she got the vaccine instead of a placebo.
On Facebook, she communicated with David Keller, a James Island resident who signed up for the AstraZeneca study on his other Charleston area website, the Medical University of South Carolina.
Keller, a 41-year-old freelance cameraman, had never participated in a clinical trial. He also had never experienced a pandemic before.
“I want to be able to live my life again,” said Keller. “I want to go to shows, go to restaurants, hug my friends and hug my parents”.

David Keller, a freelance cameraman, is enrolled in the AstraZeneca COVID-19 vaccine clinical trial at the Medical University of South Carolina. Grace Beahm Alford / Staff
He chose AstraZeneca because his vaccine does not require freezing and is cheaper than the Pfizer or Moderna vaccines, making it more likely to benefit rural and low-income residents.
He received his first dose on election day. As he felt well, he went to bed imagining that he had received the placebo.
The next day, “It hit me.”
He woke up with body aches and a fever that reached 40 degrees. He felt cold and sweated, so he recorded his symptoms in an electronic diary that participants must keep.
After that day, he felt only a slight exhaustion.
“In fact, I was a little happy to have a fever because I knew I had the vaccine,” he said. “And the side effects are a small price to pay for immunity.”
He felt good after the second dose.
“It will be a game changer,” he said. “It will end the pandemic.”

Across the city at Mount Pleasant, another man entered the same trial for the same reasons – and felt similar symptoms. Robert Donovan received his first dose at MUSC in early November.
Like Keller, he felt good the first night. The next day, he also felt tired with joint pain. He spent the day watching football, then woke up feeling normal the next morning.
He also felt basically good after the second dose.
The study lasts two years, so participants return to the test sites periodically for blood tests and consultations. Both said they will continue to wear masks and follow other guidelines if they do not receive the vaccine or recipients can still transmit the virus, an issue that remains for researchers.
“I’m going to continue as if I didn’t understand,” said Donovan, “but hoping that I understand.”
Hot spot test
On the other side of South Carolina, 73-year-old Beth Dragon was stuck at home alone in her Greenville apartment, worried about the number of people who weren’t wearing masks at the state’s COVID-19 hot spot.
In a bank, almost none. In the center, half to a third of people did not wear masks. Didn’t they care that someone like her, with asthma and other health problems, could get their virus?
Dragon, an interfaith minister, found a nearby Vitalink research laboratory serving as a test site for Moderna, another of four pharmaceutical companies with vaccines in large-scale clinical trials in the US at the time. Neither state nor federal officials were able to say how many Southern Carolinians are enrolled in the trials.
The Pfizer vaccine has since received emergency use authorization, and Moderna is due to receive it on Friday, according to press reports.
Participating in a clinical trial was nothing new for Dragon. As a child, she had tested the polio vaccine, which benefited countless people. She saw the same hope now.
“We can be stuck in our homes for another three years if people don’t speak out,” said Dragon.
She had her first chance in early August, the second near Labor Day. After the first, she was in pain, tired. For a few days, she took “some unexpected naps.”

Beth Dragon, a 73-year-old Greenville resident, poses with a photograph of herself at the age of 10. Dragon signed up for Moderna’s COVID-19 vaccine trial this summer, decades after participating in polio vaccine studies when he was 8 years old. Bart Boatwright / Special for The Post and Courier
After the second dose, the pain and tiredness worsened.
Worth it. Her 95-year-old mother lives in a nursing home and could benefit from the very vaccine that Dragon helped test for effectiveness and safety.
“I didn’t grow a tail or turn green,” Dragon said.
About 15 miles from Simpsonville, a mother who stays at home juggling a pandemic life with a husband and a kindergarten student enrolled in the same study. Janet Faulkner was also irritated by the lack of people wearing masks.
Her husband, who travels for work, said he felt safer at airports than at a gas station in Greenville. Many restaurants and stores operate at full capacity, she said, although Greenville County has registered the majority of new coronavirus cases per day across the state – by far.
“It’s mind-boggling to see,” said Faulkner.

The former professor is a master of public health. She signed up for the Moderna study thinking that since she is healthy, even if she received the placebo – as half of the participants did – she is at a relatively low risk of developing severe COVID if she contracts the virus.
She also remembered World War II, when people on the domestic front, especially women like her, started working to support the war effort.
“Get a mask and get a vaccine,” said Faulkner. “It is not much to help your fellow Americans.”

Medical workers step up
Among the larger groups that enroll in the SC Clinical Trials are medical workers who see the reality of COVID-19 and hear about the mistrust of scientists and the healthcare system.
Lauren Singleton, a first-year medical student at MUSC, always talks about the importance of trusting the medical community. As a black woman, she signed up for a vaccine test in part to “put my money where my mouth is,” she said.
Reading the 30 pages of consent and other forms, she admittedly was a little nervous: “It was very desperate.”
Still, she felt good after the first dose. After the second, she had a mild fever, tiredness and headache.
She understands that people are nervous about the speed of vaccine testing, but encourages them to consult doctors and nurses or do research on the CDC website. She does not understand the mass distrust of medical professionals.
“It has been exhausting for a lot of people,” she said.
Dr. George Minson, a semi-retired specialist in internal medicine, shared Singleton’s concerns. He and his wife, Martha, joined the AstraZeneca study together. Both are over 60 years old. George manages hypertension, cholesterol and diabetes.
He also believes strongly in vaccines and does not believe in the conspiracy theories surrounding this: “Anyone who has the opportunity to get the vaccine and refuses to do so is being foolish in the extreme,” he said.

Post-shot hours
After leaving the North Charleston lab with his bead bracelet, Ginger Bridges sat at his computer and started to work. Disappointment laced his thoughts as the hours passed.
She felt good. Certainly, she received the placebo.

Kathryn Bowis, an attending physician, performs a brief physical examination on Ginger Bridges who enrolled in the AstraZeneca COVID-19 vaccine trial. After receiving the vaccine or a placebo, Bridges will return for a second injection 29 days later. Andrew J. Whitaker / Staff
Shortly after 9 pm, she started to feel a little strange. By ten o’clock at night, she was barely able to get up, her body shaking with chills. His temperature rose. Normally happy under several blankets, she felt hot and couldn’t sleep.
“It was enough for me to confirm that it was not the placebo,” she said.
Four hours later, she started to feel better and managed to sleep. In the morning, she felt good, in addition to a slight headache.
She soon went into work as usual, never again grateful for a few hours of seasickness.