A drug that could protect high-risk Covid-19 patients from developing serious illnesses is standing on the shelves unused as a record number of people are hospitalized in the U.S.
On Thursday, public health officials at the federal and state levels beg the country to take advantage of its vast supply of treatments with monoclonal antibodies, the only available therapy that can keep patients out of the hospital.
Complete coverage of the coronavirus outbreak
“This is the first time during a pandemic that I remember when our resources far exceed demand,” said Dr. William Fales, medical director of the Michigan Department of Health and Human Services, on Thursday, during a news conference. press organized by the Department of Health and Human Services. Fales estimated that only 10% of Covid-19 patients in the state who are eligible for therapy received it.
Monoclonal antibodies are drugs made in the laboratory to mimic natural antibodies against SARS-CoV-2, the virus that causes Covid-19. They are recommended for people at high risk of becoming seriously ill with the virus, including people over 65 and people with underlying health problems.
At least one study has shown that therapy can reduce the amount of viruses in a person’s system. But no gold-standard research proves that monoclonal antibodies do provide that benefit. Most of the reports are anecdotal.
Fales said his team noted that hospitalization rates during the two weeks after therapy with monoclonal antibodies appear to be around 5 percent. That’s about half the rate of patients who received placebos in monoclonal antibody treatment studies by drugmaker Regeneron, according to the Food and Drug Administration’s emergency drug authorization.
Dr. Andrew Thomas, clinical director of Ohio State University’s Wexner Medical Center, suggested on Wednesday during a media call that the use of monoclonal antibodies eased tension in the hospital system.
Thomas said his system quickly “accelerated” the use of monoclonal antibodies. “I would like to think that this is why our hospitalizations have decreased,” he said.
Dr. Jonathan Parsons, head of monoclonal antibody treatment efforts in central Ohio, said, “Anyone who takes the test through our swab program is registered on an electronic medical record.” Parsons’ team then contacts primary care providers for positive patients, asking if they would like to refer patients for monoclonal antibodies.
New Jersey state epidemiologist Dr. Eddy Bresnitz said that monoclonal antibodies may have played a role in the recent leveling of hospitalizations for Covid-19 in the state. “It is worth the effort to obtain it,” Bresnitz said at a news conference on Thursday.
So, why are people not understanding?
Simply put, lack of time, resources and awareness.
Management obstacles
Monoclonal antibodies should be administered soon after the test result is positive. “These drugs work best when given soon,” said surgeon general Jerome Adams during Thursday’s briefing.
The two monoclonal antibody products that have been authorized for emergency use by the FDA, from drug makers Eli Lilly and Regeneron, must be administered in the first week of the disease.
But as tests are still delayed in much of the country, many patients have to wait several days to find out if, in fact, they have been infected. Simply waiting for the test results can take patients beyond the time they could qualify for treatment.
That barrier, however, should not be a factor in obtaining monoclonal antibodies, said Dr. John Redd, the medical director of the office of the assistant secretary of health and human services for preparedness and response.
“Getting these therapies doesn’t require a CRP test,” said Redd during Thursday’s briefing. (A PCR test, or polymerase chain reaction, is considered the gold standard, but it may take days to get a result.)
Instead, Redd said, “a quick test is quite appropriate.” Rapid tests can return results in minutes, but have higher rates of false negatives.
Those on the front lines of treating patients with Covid-19 say it is not so easy.
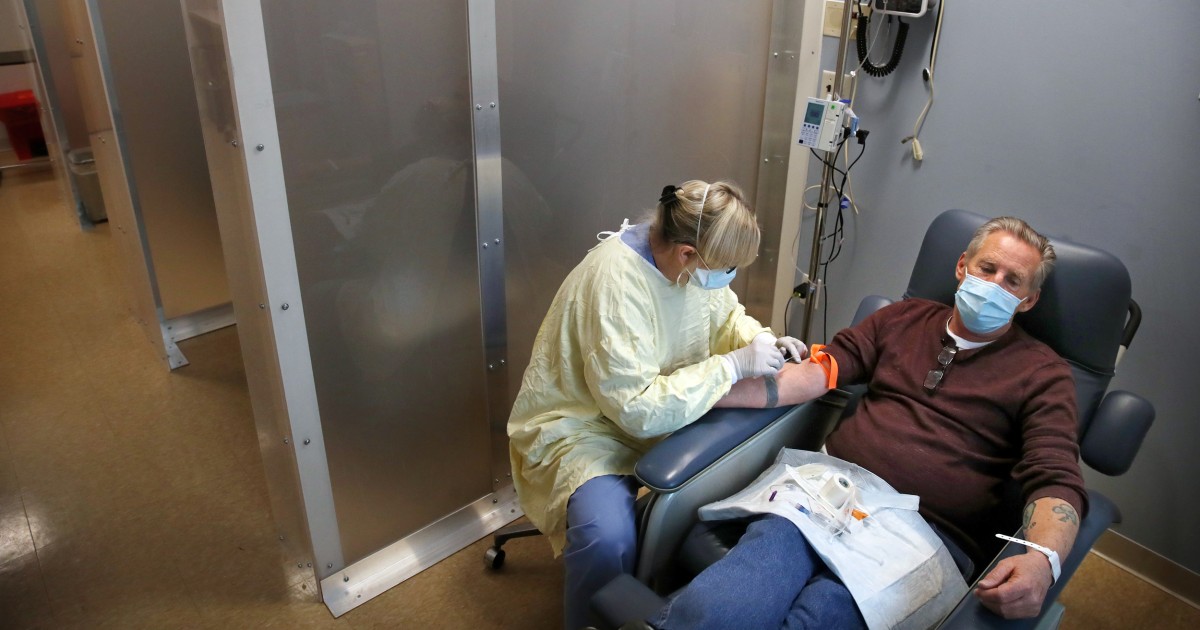
Monoclonal antibodies are administered intravenously, in an infusion of one hour, with a consultation that lasts three to four hours. Because patients with Covid-19 are contagious, they must be separated from other vulnerable patients who need outpatient infusions, such as those receiving chemotherapy for cancer.
Dr. Peter Chin-Hong, an infectious disease specialist at the University of California, San Francisco, said that some patients may refuse treatment simply because they are feeling better. But this can be a mistake. It was clear that some patients may feel better before they suddenly get worse.
For many others, logistical problems get in the way.
Public transport and rides, like Uber, are out of the question for those with Covid-19 active. In addition, Chin-Hong said, some patients simply cannot afford three hours out of the day outside of work or family obligations.
Chin-Hong estimates that his health care system used less than 20% of the monoclonal antibodies in stock.
In addition, special infusion centers must be created and staffed. Some say it is an irrational demand on health systems that are already overwhelmed.
“If we had this pandemic under control, we could establish infusion centers. We could establish rapid tests. But we don’t have these resources,” said Dr. Pieter Cohen, who is an associate professor at Harvard Medical School and a physician at the Cambridge Health Alliance Respiratory Clinic. near Boston.
“We are completely inundated with sick patients,” said Cohen.
Chin-Hong agreed. “These patients are generally well and you want to focus on sick patients,” he said.
“I think that’s where people’s minds are – particularly in California now,” he said. The state has seen an increase in cases of Covid-19 recently. In the state’s most populous county, Los Angeles, an average of 10 people test positive for the virus every minute.
The obstacles are not lost by at least some of those leading the federal response. “We recognize that the health care system is very stressed,” said Dr. Janet Woodcock, therapy leader for Operation Warp Speed, during the media call on Thursday.
“On the other hand, if we don’t do this, the likelihood is that we will have even more overburdened hospitals and healthcare professionals,” said Woodcock, adding that his team feels that efforts to create these infusion centers are “worth it” to reduce costs. burden on health systems.
Some autonomous kidney dialysis centers across the country have announced that they will begin administering monoclonal antibodies to Covid-19 patients during shifts configured only for those patients. Covid-19 has been shown to be especially terrible for patients with kidney disease.
Download the NBC News app for complete coverage of the coronavirus outbreak
Another factor may be the lack of knowledge, both from patients and professionals, that treatments are available.
During a press conference on Tuesday, Health and Human Services Secretary Alex Azar put the burden of seeking monoclonal antibodies on patients, who “should ask their doctors or health professionals why they are not receiving these antibody therapies. “.
However, the HHS online tool offers little help to those trying to find monoclonal antibody resources. The site has no data on people in at least 31 states, including Alabama, Kansas, Michigan, New Jersey, New York, North Carolina and Washington.
An HHS spokesman said on Thursday that the team is working “as soon as possible” to update the site and that it expects more resources to be available next week.
Follow NBC HEALTH on Twitter & Facebook.