Gene editing technologies increase the chance of attacking the root cause of certain inherited human diseases. Writing in Nature, Koblan et al.1 report the use of such technology in mice that provide a model for a human accelerated aging disorder.
Aging is influenced by several factors – some external, some specific to organs and others systemic, affecting the entire body. It is one of the main biological processes for which the main cause or causes are not fully known. Often, the mechanisms underlying a biological process are revealed by an analysis of genetic mutants, and mutations in systemic aging factors are associated with accelerated aging processes that affect multiple organs. Most disorders of premature aging point to problems in the maintenance and integrity of DNA as the underlying cause. People with Werner or Cockayne syndrome, for example, have defects in different mechanisms that affect the stability of the genometwo.
Generally, conditions of premature aging exhibit accelerated aging of a subset of tissues. The most well-known of these disorders is the Hutchinson-Gilford progeria syndrome, which is often called just progeria. Children with this condition look healthy at birth. But, around one year of age, symptoms start to appear, such as growth failure, skin abnormalities and hearing loss. The characteristics of premature aging increase over time, resulting in striking features of old age that include wrinkles, loss of fat under the skin, joint stiffness and musculoskeletal abnormalities. However, these children keep a nervous system functioning normally, emphasizing the specific nature of the syndrome organ. There is no cure for progeria, and affected individuals usually die at around 14 or 15 years of age as a result of conditions such as atherosclerosis, severe cardiovascular complications or stroke3.
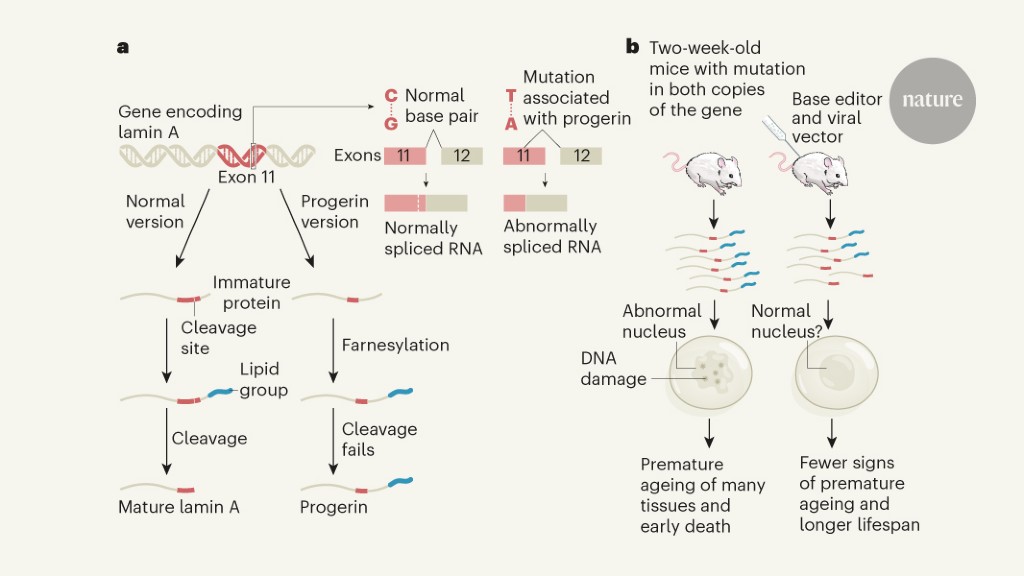
Progeria is caused4 by mutating a single base (a cytosine with a thymine mutation) in one of the two copies of the gene encoding the lamina A protein (Fig. 1). This protein is a structural component of the outer edge of the cell’s nucleus. The mutated version of the gene leads to abnormalities during the splicing process that occurs during gene transcription. As a result, a shorter-than-normal version of slide A, called progerin, is produced. The maturing progerin and lamina A undergo a modification called farnesylation, in which a specific lipid group called farnesyl is linked to the protein. As blade A matures, this lipid-modified region of the protein is removed in an enzyme-mediated cleavage event. However, progerin remains farnesylated because it lacks the amino acid residues that provide the usual cleavage site. Farnesylated progerin accumulates and impairs the normal role of blade A, thus disrupting nuclear shape, stiffness and function.
Nuclear malformations due to progerin are particularly apparent in organs and tissues subject to mechanical stress, including the skin and the cardiovascular system. The parts of the body that are subject to high mechanical stress correspond to Organs most affected organs in progeria, and the nuclear deformations that arise from stress activate the response to DNA damage5,6. Nuclear weakening induced by progerin can result in genome instability under mechanical stress if several delicate processes in the nucleus are disturbed. This would be consistent with the above normal levels of DNA damage and chromosomal aberrations found in cells of people with progeria and in mice that provide a model for the syndrome.7, classifying progeria among other disorders of premature aging associated with genomic instability.
Attempts to find treatments for progeria initially focused on trying to reduce the accumulation of farnesylated progerin8. The compounds that generated interest included those with reported anti-aging activities, such as metformin and rapamycin – which can affect progerin splicing and turnover, respectively. The most advanced drugs, in terms of clinical use, are the inhibitors of the enzyme farnesyl transferase, which reduce the accumulation of farnesylated progerin. One of them, telafarnibe, was approved in November 2020 by the US Food and Drug Administration. This is the first licensed therapy for progeria. However, treatment only partially relieves the syndrome8.
The most rigorous approach to combat progeria would be to directly correct the genetic defect. In 2019, two teams reported using the CRISPR-Cas9 gene editing method to repair the associated mutation in the gene encoding blade A in mice9,10. This treatment alleviated the decline in health normally expected in such animals and extended their lifespan, compared to those whose mutation was not corrected. CRISPR – Cas9 targets a specific genome sequence through a process aided by a guide RNA sequence that helps ensure that editing takes place at the desired location. However, unwanted changes can arise – either from the formation of double-stranded DNA breaks that occur during this editing process, or from off-target edits of sequences that are similar to the sequence of interest. The possibility of such unwanted events would require extreme caution in any potential clinical implementation.
Koblan et al. introduce the use of an editing approach that can offer a way forward. The authors took advantage of tools known as base editors11. Like CRISPR-Cas9, they can alter a single base at a specific genomic site, such as where the mutant thymine is paired with adenine in the gene encoding lamina A. However, one difference is that the base editors do not cleave the structure of DNA phosphate when targeting the nucleotide, so double-stranded DNA breaks are not generated. Instead, the approach chemically modifies the target nucleotide12. Adenine-based editors convert adenine, through an intermediate called inosine, into guanine during DNA replication. The need for DNA replication can present a problem if you try to use this method to target mutations in other diseases in which cells that do not divide, such as those of the nervous system, are the main target of interest.
Koblan and colleagues used an adenine-based editor, transferred to cells using a virus called lentivirus, to target the mutation in the gene encoding lamin A in cells of people with progeria. The repair occurred in 90% of all cells. The mutation correction resulted in normal lamina A splicing, reduced progerin expression and corrected abnormalities in the nuclear form. Minimal off-target editing was observed.
Using a mouse model of progeria, the authors delivered the basic editor using adeno-associated viruses. After a single injection of the editor near the eye socket, or in the abdominal cavity, of rats up to two weeks old, the authors observed the targeted repair of the gene that codes for blade A in many organs. This happened mainly in the liver and heart, but it also occurred, to a lesser extent, in the muscles, the aortic artery and bone tissue.
Although most of the major organs affected in progeria showed only a modest level of genetic correction and a reduction in progerin, a notable increase in the level of lamina A was seen as a consequence of treatment. Crucially, compared to model animals that have not undergone genetic editing, those that have received the basic editor have aged with noticeably fewer abnormalities in the cardiovascular system that are generally life-limiting. These animals also had greater vitality (a better ability to move and a better overall appearance) and a statistically significant life span.
By directly addressing the root cause of the disease, the basic edition can offer great advantages over current drug-based therapeutic strategies. Many key questions still need to be answered, however, before people can benefit from the introduction of this technology. For example, what is the base editor’s ideal distribution mediated by adeno-associated viruses or other delivery methods? And which organs can be targeted? The strategy of injecting adenosociated viruses was less efficient in reaching the skin than in other organs of mice.
To what extent can the genetic defect be corrected? The high efficiency of editing can be crucial, especially for efforts to treat other diseases. Previous attempts11,12 using gene editing to treat defects underlying Duchenne muscular dystrophy and rare liver disease has had only limited success. However, the work of Koblan and colleagues indicates that the correction does not need to reach 100% efficiency to provide positive benefits, opening the possibility to reconsider this approach for some other diseases as well.
Another important question is whether an immune response can develop and reach the components of the editing system. Such a response may result in inefficient treatment if cells that house editing components are selectively eliminated11.
What about long-term considerations? For example, would a single publisher management be sufficient? And what would be the best age for treatment to be administered? Progeria is diagnosed relatively early in life, compared to many other diseases for which the basic edition is a possibility. A treatment age of two weeks for mice is therefore much less than the equivalent age for humans, at which many diseases are diagnosed. In addition, with animal testing, it is difficult to compare human age equivalent to a mouse of a certain age. Finally, how would the current drug therapy available for progeria fit the potential for repair by basic edition in a treatment plan?
If the basic edition is used to treat human diseases, the safety of such an intervention must be guaranteed. If possible, and if this method successfully repairs the alteration that causes progeria in crucial tissues, such an approach holds great promise as a way to prolong health, extend life expectancy and improve the quality of life of those who have this mutation.