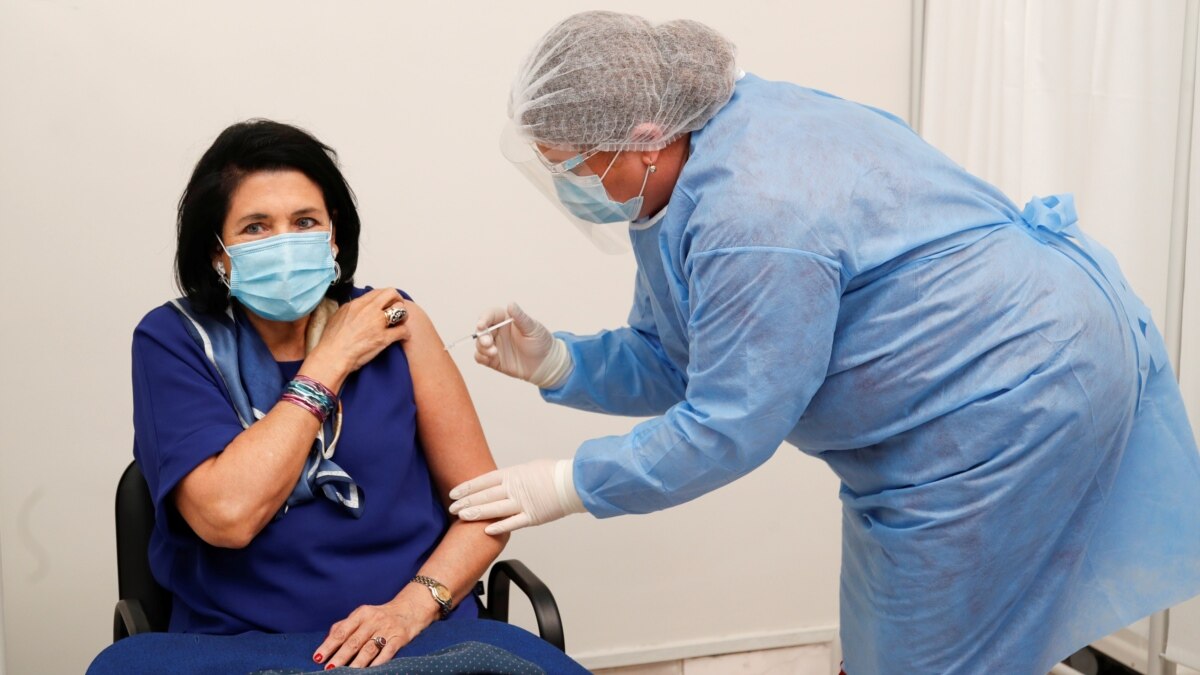
The European Union drug control agency says the AstraZeneca COVID-19 vaccine is safe.
The European Medicines Agency (EMA) on Thursday announced its findings from an examination of the medical records of millions of people who received the drug. The test began after reports that a small number of people experienced blockages in the blood system, or clots, after receiving the vaccine. As a result, several European countries suspended the use of the AstraZeneca vaccine last week.
EMA spoke after an extraordinary meeting to discuss concerns among its member countries and others.
Emer Cooke leads the EMA. She told reporters on Thursday: “Our scientific position is that this vaccine is safe and effective option to protect citizens against COVID-19. “
Cooke added, “If it were me, I would be vaccinated tomorrow.”
However, she said the agency “cannot definitively rule out a link” between blood clots and the vaccine.
The EMA said it analyzed about 25 cases of rare blood clots from 20 million people who received the vaccine by March 16. The agency said that “a causal relationship with the vaccine is not proven, but it is possible”, adding that the problem must be studied further.
The agency said the number of blood clots reported was less than expected in the general population. This led to the conclusion, reported the EMA, that “there is no increase in the overall risk of blood clots”.
Resuming vaccination programs
About 13 European nations have suspended the use of the AstraZeneca vaccine after reports of possible blood clots related to the vaccines.
On Thursday, Italian Prime Minister Mario Draghi welcomed the decision by the EMA. He said Italy would restart AstraZeneca’s vaccines as early as Friday.
When the AstraZeneca vaccine was approved for emergency use in Britain last December, the injection was described as the “vaccine for the world”. It costs much less than Pfizer and Moderna vaccines. It also does not require extreme cold storage, making it easier to use in countries with limited resources.
COVAX is an international program to distribute coronavirus vaccines to people around the world. It expects to provide at least 2 billion shots, mostly from AstraZeneca, to poor countries around the world.
Two other vaccines have been approved for emergency use in Europe. One is from Pfizer-BioNTech and the other is a Moderna product. In the United States, health officials have approved vaccines from Pfizer-BioNTech, Moderna and Johnson & Johnson for emergency use.
AstraZeneca is planning to seek approval from the US government in the coming months.
Michael Head is a leading researcher at the University of Southampton in Great Britain. He fears that people may be less willing to receive the AstraZeneca vaccine when the suspension ends. “This is a time when we need to stop the virus circling“, he said,” to reduce the chances of more variants emerging.“
Speaking to reporters on Thursday, the director of the World Health Organization for Europe, Hans Kluge, said that countries should continue to use the AstraZeneca vaccine.
He added: “We need to renew trust, if lost, to restore it – especially for AstraZeneca. “
I’m Caty Weaver.
Hai Do wrote this story for Learning English. Caty Weaver was the publisher.
______________________________________________________________
Words in this story
option – n. a choice or possibility
Circular – v. to move from group to group
variant – n. a virus that is different in one person than in the other
emerge – v. become known
trust – n. feeling or belief that something is good